Drug Research Ethics Committee (in Spanish, Comité de Ética de la Investigación con medicamentos, CEIm)
It is the independent body, made up of healthcare professionals and non-healthcare members, responsible for ensuring the protection of the rights, safety and well-being of subjects participating in a clinical trial and for providing public assurance in this regard.
Its purpose is to supervise and monitor clinical trials on an ongoing basis in order to ensure that they comply with current regulations on methodological and ethical aspects.

FIVO CEIm
President:
Esteban Morcillo Sánchez (Clinical Pharmacologist).
Vice-President:
Technical Secretary:
Carlos Andrés Blasco (Pharmacist Specialising in Hospital Pharmacy).
Board Members:
- Ismael Pastor Climente (Pharmacist Specialising in Hospital Pharmacy).
- Joaquín Gavilá Gregori (Doctor Specialising in Oncology).
- Ángel Luis Guerrero Zotano (Doctor Specialising in Oncology).
- Lourdes Bello Luna (Graduate in Law).
- Mª José Ortega García (Graduate in Law).
- Julio Tudela Cuenca (Pharmacist Specialising in Clinical Analyses).
- Jose Luis Trillo (Primary Care Pharmacist).
- Luis Gómez de Membrillera Quesada (Specialist in Family and Community Medicine).
- David Hervás Marín (Graduate in Biostatistics).
- Josefina Balaguer Cusi (Non-professional Member).
- Esteban Mesas Plaza (Graduate in Nursing).
- Elena Oliete Ramírez (Doctor Specialising in Family and Community Medicine).
- Antonio José Revert Ventura (Doctor Specialising in Radiology).
- Miguel Ángel Sanz Alonso (Haematology Specialist).
CEIm Technical Secretary Coordinator:
Bernat Navarro Aguir (Graduate in Pharmacy)
The documentation to be submitted for the evaluation of clinical trials with medicinal products/medical devices is as indicated in the “Document of instructions of the Spanish Agency of Medicine and Medical Devices for conducting clinical trials in Spain (in Spanish)” and in the “Memorandum of Collaboration and Exchange of Information between the Spanish Agency of Medicine and Medical Devices and the Ethics Committees for Research on medicinal products (a translation from Spanish)“.
Part I documents to be sent to the AEMPS (Spanish Agency for Medicine and Medical Devices) and the CEIm *
- Submission letter
- Application form
- Authorisation from the sponsor to the applicant, if applicable
- Protocol summary
- Protocol
- Researcher’s manual or data sheet for an investigational medicinal product
- Data sheet or researcher’s manual for non-investigational (auxiliary) medicinal products
- Scientific Advice and Paediatric Investigational Plan
.
Part II documents to be sent only to CEIm*.
- Documents related to the procedures and material used for recruiting subjects.
- Information sheets and informed consent documents
- Suitability of the researcher
Documentation to be provided by each centre:
- Principal Investigator’s Curriculum Vitae
- Adequacy of facilities
- Conflict of interest of the Principal Investigator
- Principal Investigator’s Certificate of Good Clinical Practices
- Proof of insurance coverage or financial guarantee
- Economic Report
- CEIm fee invoicing data form. The invoice is issued after completing the evaluation process. The complete document must be submitted together with the application. If an exemption is requested, see the conditions in the document.
For clinical trials with medicinal products, the sponsor must submit the documentation through the Clinical Trials Portal of the Ministry of Health, Social Policy and Equality.
For clinical trials with medical devices, the sponsor must submit the scanned documentation by e-mail to ceim@fivo.org. In this e-mail, the sponsor must indicate the title of the study, the protocol code, the sponsor, and the Principal Investigator of FIVO (if applicable).
The sponsor must submit all scanned documentation (PDF) by e-mail to ceim@fivo.org.
LOCAL DOCUMENTATION ( DOWNLOAD EOm REQUIREMENTS ):
For EOm (Observational Studies with Drugs) that, at the time of submission, do not have a favourable opinion as issued by the Drug Research Ethics Committee (CEIm), the following documentation must be submitted in PDF format for review by the CEIm-FIVO:
The sponsor must submit all scanned documentation by e-mail to ceim@fivo.org
GENERAL DOCUMENTATION: (Only required in PDF format)
- Cover letter (signed). The letter must specify the sponsor, the title of the study, the code, the rincipal investigator at the centre and the list of the documentation attached to the application.
- Internal study and/or university registration form (signed). To be completed only for internal and/or university studies.
- Complete protocol, adapted as far as possible to the structure and content detailed in Annex I of the Spanish “Royal Decree 957/2020, of 3 November, regulating observational studies with drugs meant for human use”. It may be accepted in English, with a summary in Spanish. Its version and date shall be indicated.
- Information sheet for participating subjects and informed consent form, or justification for exemption. CEIm-FIVO form is available. Version and date to be noted.
- Case Report Form (CRF).
- General economic report. Financing sources for the study and compensation foreseen for the participating subjects and researchers, if applicable. In the case of non-commercial clinical research, the sponsor must submit a declaration signed by the sponsor and the coordinating researcher that the study complies with all the conditions referred to in paragraph e) of article 2.2 of Royal Decree 1090/2015, of 4 December.
- List of researchers from each of the healthcare centres in which the study is proposed to be conducted and the number of participating subjects intended to be included in each autonomous community. If the study is planned to be carried out in other countries, a list of the countries must also be included.
- Responsibility delegation agreement (CRO). If the application is not submitted by the sponsor, a document indicating the tasks delegated by the sponsor to the person or company acting on his or her behalf must be included (if applicable).
- Evidence of approval of the protocol by the relevant body, in the case of a study imposed on the marketing authorisation holder of a medicinal product by the national competent authority or the European Commission (where applicable).
- Invoice request / Application for evaluation fee waiver (signed).
LOCAL DOCUMENTATION ( To be sent to the CEIm Secretariat duly SIGNED, according to the CEIm-FIVO’s own form):
NOTE:
- EOm promoted by an external Sponsor : The FINCIVO Coordinator (coordinacion@fincivo.org) will be asked to manage this documentation and send it to the Sponsor.
- EOm promoted by FIVO’s PR: The Researcher will manage it him/herself.
- Commitment of the Researcher and authorisation of the Head of Service.
- Agreement of involved services. Extraordinary tests and/or collaboration with other services (only when applicable).
- Form on general characteristics of the study.
- Questionnaire for studies with €0 economic report. Only in the case of studies with €0 economic report.
- Confidentiality agreement for trainees. Only in the case of university studies.
- GDPR clause. Only in the case of studies at the university level.
The observational study with drugs (EOm) cannot begin at the centre until the corresponding management approval has been obtained (if the sponsor is FIVO staff), or the corresponding contract has been signed (if the sponsor is external to the centre). To this end, it is a minimum requirement to provide proof of having received the authorisations for the funding requested. Both the management approval and the contract must be requested at the following e-mail address: contratosestudios@fivo.org
The sponsor must submit all scanned documentation (PDF) by e-mail to ceim@fivo.org.
DOCUMENTATION TO BE SUBMITTED ( DOWNLOAD REQUIREMENTS FOR RESEARCH PROJECTS [PDF in Spanish]. ):
The following documentation must be submitted IN PDF FORMAT for review by the REC-FIVO:
GENERAL DOCUMENTATION
1. Cover letter (signed). The letter must specify the sponsor, the title of the study, the code, the principal investigator at the centre, and the list of the documentation attached to the application.
3. 1 Protocol summary.
5. Case Report Form (CRF).
7. BIOBANK sample availability report (only if samples from the biobank are required).
8. Certificate of Civil Liability Insurance (Art 18 of Law 14/2007) (if applicable).
9. Responsibility delegation agreement in the case of CROs (if applicable)
10. Proof of payment (if applicable). Invoice request / Application for evaluation fee waiver.
LOCAL DOCUMENTATION (send an original according to the CEIm-FIVO’s own template):
1. Commitment of the Researcher and authorisation of the Head of Service .
2. CV of the research team (abbreviated format recommended).
3. Agreement of involved services. Extraordinary tests and/or collaboration with other services (only when applicable)
4. Principal Investigator’s Report.
5. Questionnaire for studies with €0 economic report. Only in the case of university studies.
6. Confidentiality agreement for trainees. Only in the case of university studies.
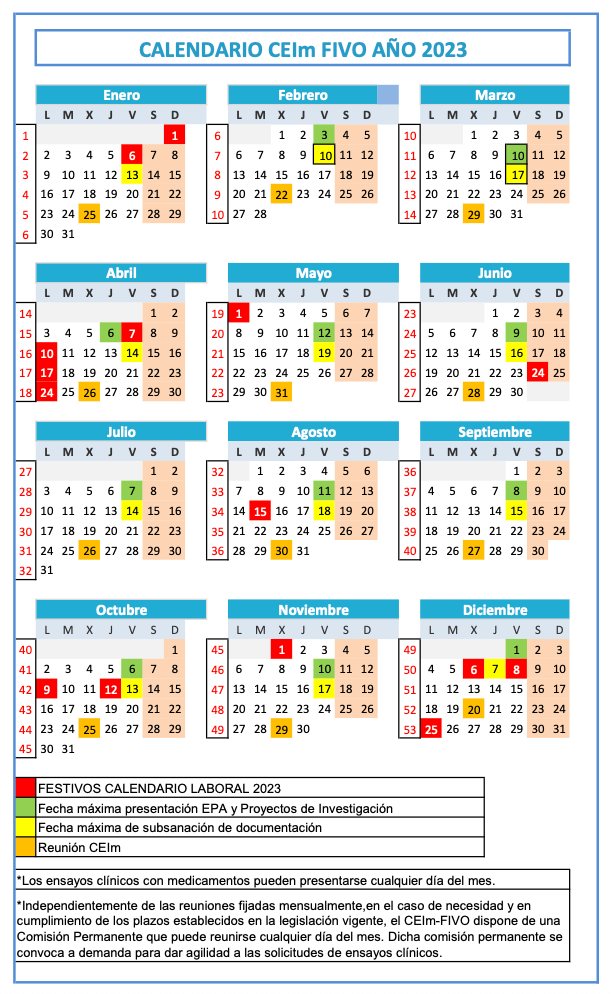
Submission period: From the 1st to the 10th of each month. Documentation may be corrected until the 15th.
CEIm Technical Secretariat
Bernat Navarro, Beatriz Llatas and Carmen Taltavull
E-mail: ceim@fivo.org
Telephone: (+34) 960 694 674 | (+34) 961 104 673 | (+34) 961 114 095
Address: Gregorio Gea, 31 CP 46009 – VALENCIA
Edificio D, 1ª Planta Secretaría Técnica CEIm
Management of Clinical Trial contracts
At the IVO Foundation, the Medical Director is the person authorised to sign the document of facilities’ suitability.
To make a request to process the signing of this document, the sponsor/CRO can send an email to: contratosestudios@fivo.org
The following documentation and information must be attached to the request (DOWNLOAD REQUIREMENTS FOR FACILITY SUITABILITY MANAGEMENT IN PDF FORMAT):
- Protocol in Spanish or, failing that, the protocol in English plus a synopsis in Spanish.
- Facility suitability model completed with the data of the study indicating the services involved that will participate in the study.
- General Economic Report
In addition, the following data must be indicated in the application e-mail:
- Name of the principal investigator
- Service of the Hospital where the study will be carried out
- Whether they need the document with an original signature or if a PDF scan is sufficient.
Clinical trial contracts signed with the Instituto Valenciano de Oncología Foundation shall be managed in accordance with the instructions described below.
The contact e-mail address for contract negotiations is contratosestudios@fivo.org
Documentation and information to be provided by the sponsor to complete the centre’s internal file (DOWNLOAD REQUIREMENTS FOR CLINICAL TRIAL CONTRACT MANAGEMENT [PDF].):
1. Protocol in English or Spanish
2. Summary of the protocol in Spanish.
3. Patient information sheet and informed consent.
4. Annex 1A (if available at the time of signing)
5. Certificate of Insurance Policy. The following information must be included:
Centre: Fundación Instituto Valenciano de Oncología
Address of the Centre: C/ Profesor Beltrán Báguena, 8, 46009 Valencia
Foundation: Fundación de Investigación Clínica del Instituto Valenciano de Oncología (FINCIVO)
Foundation’s Address: C/ Ricardo Micó, nº 3 – Local nº 1 46009 Valencia
Principal Investigator.
7. Powers/delegation (Contract Research Organization, CRO). Justification of the representation of the signatory parties to the contract.
8. Opinion of the CEIm (if available at the time of signing)
9. AEMPS auhorisation (if available at the time of signing)
10. Principal Investigator’s CV
12. Name of the CEIm and status of the application to CEIm and AEMPS . Please complete the attached form.
LOCAL ECONOMIC REPORT
Concurrently, the annex to the local economic report is to be negotiated with Bernat Navarro bnavarro@fivo.org (PHONE +34 960 694 674). Depending on how the contract will be signed, one of the following forms should be used:
– Annexed Form on the Internal Economic Report FIVO contract (in Spanish).
– Annexed Form on the Internal Economic Report FIVO contract (bilingual).
THESE ARE TWO INDEPENDENTLY MANAGED PROCESSES
– The body of the contract is negotiated via contratosestudios@fivo.org (Do not copy this address in the e-mails addressed to the negotiation of the local economic report)
– The local economic report is negotiated directly with bnavarro@fivo.org (Do not copy this address in the e-mails addressed to the negotiation of the body of the contract). Once the local economic report annex is closed, it can be included in the contract.
Following the publication of Decree 73/2009, of 5 June, of the Consell, which regulates the management of clinical trials and observational post-authorisation studies with medicine and medical devices. [2009/6667], the following contract templates will be used:
- FIVO SAMPLE CLINICAL TRIAL CONTRACT (in Spanish)
- FIVO SAMPLE CLINICAL TRIAL CONTRACT (bilingual)
- FIVO SAMPLE PAS CONTRACT (in Spanish)
- FIVO SAMPLE PROJECT CONTRACT (in Spanish)
In the event that a clinical trial is extended, the Principal Investigator is replaced, or there is a major modification that entails an increase or decrease in the initially planned cost of the clinical trial, an addendum to the contract shall be made.
The following addenda templates will be used:
*In the event that it is necessary to sign a bilingual form of the addendum, a sworn translation must be provided, enclosing a certificate from a sworn translator accredited by the Ministry of Foreign Affairs.
FIVO Research Secretariat
Bernat Navarro, Beatriz Llatas and Carmen Taltavull
E-mail: contratosestudios@fivo.org
Telephone: (+34) 961 104 673 | (+34) 961 114 095
Address: Gregorio Gea, 31 CP 46009 – VALENCIA
Edificio D, 1ª Planta Secretaría Investigación FIVO
Clinical trials at the IVO
The IVO has participated in the development of most of the new cancer drugs marketed in the last 20 years and currently has one of the most clinical trials on cancer drugs in all of Spain.